

|
Member discussion regarding the methods, varieties and merits of growing tomatoes.
|
 |
|
|
Thread Tools | Display Modes |
|
|
#1 |
|
Tomatovillian™
Join Date: Nov 2011
Location: Long Island NY
Posts: 1,992
|
Grafting Gallery Photos
http://bit.ly/1k1bL50 It took me seemingly forever to get this write up into a cohesive form. “Lifes Rich Pageant” kept getting in the way. So, here it is and I hope it is useful. Need to lead with a hearty “many thanks” to all the fine folks who graciously shared their experiences in the “Best grafted tomato varieties?” thread. Definitely worth a read through if you are going to try your own. http://67.23.252.182/~tomatovl/showthread.php?t=26079 Also need to give a big shout out to David Francis at OSU for graciously replying to my multiple emails on the subject of glue grafting while he no doubt had better things to do.  I can’t take credit for the first successful glue graft on Tomatoville. That distinction goes to Anne (aclum) using Loctite brand gel applied with a toothpick. Anne also came up with a “grafting jig” which is a great innovation. I will elaborate on the jig later. Results: 25 successful grafts out of 36 attempts with glue or 69%. To break it down further: Round 1 – 11 for 18 (61%) Round 2 – 14 for 17 (82%) I did do one graft (not counted in the above totals) with a 2mm clip which was also successful. Variety Totals Cherokee Purple - 9 Stump of the World – 5 Goose Creek – 1 Japanese Trifele Black – 3 Vorlon- 7 Rootstock – Maxifort - which gets high marks for vigor. I probably should have kept track of the attempts per variety but it did not occur to me at the time of grafting so I only have a total successful count. Background – I was aware of grafting but not necessarily that interested. Growing in a soilless mix, the primary reason for grafting, mitigating soil borne disease, isn’t really an issue in my opinion. That kinda changed last August as I happened across Camochef’s “Going out on a limb here!” thread. Camo – “Well I just finished eating the best tasting tomato I've ever tasted...I know, this is a bold statement, but this plant has been unreal since it went into the ground and it's now producing something special.” http://67.23.252.182/~tomatovl/showthread.php?t=24399 When someone who has grown as many tomatoes as Camo makes that kind of statement, I tend to sit up and start paying attention. Which brings us to the secondary reason for grafting – Vigor. Improved production, size and taste? Really?? Yes please…. Whether I get any of them remains to be seen, but it was certainly worth a shot. So the research and plotting began. Shortly thereafter I also came across the concept of grafting with glue, mainly via the video from David Francis’ group at OSU as they are the only one utilizing the method. http://oardc.osu.edu/graftingtomato/...ods/index.html also on Youtube - http://www.youtube.com/watch?v=5Fd6tBQTTAg Which begged the question – Why is no one else doing this? It also led to my first preconceived notion about glue grafting: Preconceived Notion 1 – Grafting with glue would be much easier than using grafting clips. (It wasn’t) I will go ahead and list my second preconceived notion which did not come until later after researching cyanoacrylate glue: Preconceived Notion 2 – I would get different results depending on the brand and or type of cyanoacrylate used. (I did not) Types of Glue I have a lot more research on the subject but here is the gist (for grafting purposes you can essentially forget it and skip down if you prefer): 3 main types of cyanoacrylate: 1. Ethyl cyanoacrylate (short chain) has an exothermic reaction, strongest bond. Can be an irritant. - Brands - Krazy Glue, Loctite, Future Glue, etc. 2. Butyl cyanoacrylate (medium chain) is bacteriostatic, no exothermic reaction, weaker bond than Ethyl. Used in some medical glues, more common as a veterinary glue, i.e - patching up a dog's foot pad, declawing procedure. - Brands -Vetbond, Veterinary glue. Also used in some dental glues. 3. Octyl cyanoacrylate (longer chain) is bacteriostatic, no exothermic reaction, weakest bond. Used in medical glues. Brands - Octylseal, Dermabond. Glues  My preconceived notion was based on the thought that the Ectyl types (store brands) would have higher failure rates due to the heat and irritation reactions. Not enough data for any sort of true statistical analysis but I did not observe any real differences between the brands used or the type of glue. I obtained 2 types of Ectyl – Krazy Glue and Future Glue; 1 type of Butyl – Vetbond; and I type of Octyl - Demabond. The intent was to try them all over a few rounds of grafting. The Ectyl, off the shelf brands are the clear winners on a cost basis. They are inexpensive and several come prepackaged with their own brush. OSU prefers Krazy Glue, I preferred Future Glue. My preference was simply because the brush length was shorter and the bristles softer and I felt I could more easily manipulate it around the plant. Vetbond – Very viscous. Not a great applicator. I thought the viscosity was going to present a problem due to “seepage” into the graft cut after Round 1 but I was unable to discern any problems with graft success. Demabond – I intended to use it in Round 2, but after my initial successes in Round 1, it never made it out of the package so it will be saved for next season. OSU and Glue Grafting David Francis started its use with Tomatoes – “I came up with the idea after learning about the use of glue for medical (human and vet) uses in place of sutures. Then, I learned that the Japanese have been using adhesives for plants. There are several publications that talk about this for use in Cucurbitaceae. So the idea is not original.” More from David – “For top grafting/tube grafting (both scion and RS cut with a 30 degree angle) We get higher % graft success using cyanoacrylate glue. Our glue of choice is "Krazy Glue", and we prefer the applicator that comes with its own brush.” I also asked him about success rates with grafting methodologies - “We have not done side to side comparisons with cleft grafting and glue-based top grafting. My sense is that cleft grafting gives us higher take. Several of the RS we work with have "incompatibility" issues, and we use glue to raise success to an acceptable level. For example, the bacterial wilt resistant line Hawaii 7998 and hybrids with this line give us poor success with both cleft graft and tube grafting. We can raise this to ~70% or greater with glue.” And my favorite – “With glue grafting, you must be very careful not to get glue on the surface you are trying to join. The glue goes around the outside.”  Maxifort Rootstock – It gets high marks for vigor and it also seems to get a lot of commentary with respect to uneven germination and growth habit. I had a somewhat different experience – 37 out of 40 seeds germinated and very even growth. I pre-germinate all of my seed though, and used temperature to mitigate the growth rate which I am sure had something to do with it.  Procedures My set up- Basement Boiler room with standard fluorescent lighting (6500 bulbs) on shelves as well as my workbench area. Additional shelves with identical lighting in my unheated garage. Pre-germinated 3 batches of both rootstock and scion seed spaced over the space of 6 days. My intent in doing so was to better manage the germination and potting process and less about using germination spacing to try and achieve “matched” plant sizes. 40 Maxifort in total with 37 of the 40 germinating. 65 scion seed with 58 germinating. Potting blocks – Germinated seeds go onto ¾ inch blocks and then potted up to 2 inch blocks when seedlings reach 2 to 3 inches in height. All of my grafting was done in the 2 inch blocks. I also use the 4 inch blocks and the plants can remain in this size for a very long time until planting. Damping off – I make the ¾ inch blocks a day in advance of use and spray them and their associated containers with a 3% solution of hydrogen peroxide. I have had good success using Hydrogen Peroxide in this manner. Matching rootstock to scion – In order to get as many close matches in size as possible I used temperature. My basement room temps in March fluctuated between 65 and 78 degrees depending on the boiler being off or on. Humidity in the 50% to 60% range. My garage temps fluctuated between 35 and 50 degrees depending on the time of day. I ran an electric oil-filled heater on more than a few nights when the outside temperatures got down in the neighborhood of freezing. They stayed in the basement until true leaves were forming/ formed before going to the garage. I sorted and resorted plants on several occasions to “like size” in the trays and moved them back and forth between the 2 locations over a several week period. Great success using temperature to achieve size matches – If possible I recommend it. Side bonus – they were also getting the “cold treatment”. First seeds were started 3.10. 13 First round of grafting 3.31.13 Second round of grafting 4.11.13 – I delayed this somewhat due to time constraints and I wanted larger plants. Grafting Tools Wilkinson Sword Razor Blades – snapped in ½ lengthwise Note – I really liked these blades as a cutting tool. They come individually wrapped making them easy to snap in half in the wrapper. Very thin and sharp, nice clean cuts. Cyanoacrylate Glues Common 1020 tray with associated humidity dome Heat mat and thermostat Grids below are optional but I like them for potting block use Humidity grids 1020 mesh grids My chamber setup: I had 2 standard 10x20 trays as humidity chambers set up on the same 20x20 hydrofarm heat mat. http://www.hydrofarm.com/product.php?itemid=3351 along with their digital controller. http://www.hydrofarm.com/product.php?itemid=3372 Used their vent-able humidity domes as well. http://www.hydrofarm.com/product.php?itemid=8273 Inserted in the bottom were these humidity grids. Could easily use these in the bottom of a tote as well to elevate the seedlings. http://growhome.com/Propagation/Tray...y-Grid/GH2211/ Temp set at 78 deg with the heat probe inserted into an empty potting block kept both domes at a constant 80 to 82 deg. The humidity grids allow you to put water in the bottom of the trays. This kept the RH above 90% and really required no monitoring. I used 1% H2O2 in lieu of straight water and really thought it helped with damping off. (I had none) I have this inexpensive humidity/temp meter that I tested it with on setup. http://www.thermoworks.com/products/humidity/rt819.html Note on humidity meters - Unless you have a very expensive meter, they all tend to saturate after a period of time so unless you are going to go expensive, go cheap and spot test occasionally. Note on the trays - you can buy "quad thick" 10x20 trays, I have several and love them, but the standard humidity domes don't fit properly on them so use the cheap single trays if you go the 10x20 route. Grafting Procedure Many different and successful methods used by both the pros and the folks here at Tville. What I took away from my reading on the subject is that there is no one “right” way to do it and success can be achieved using various methodologies. I viewed glue as merely a replacement for clips in the grafting procedure. Other than changing the “cut angle” when using clips, I would do everything the same. My keys for success in no particular order: Hygiene Consistent Temperature Consistent Humidity Consistent Stem Diameters Closely matching cuts Set up cleaned and sterilized humidity dome positioned on Heat mat in advance. Thermostat probe stuck into an unused 2 inch potting block. (soil in a standard cup would work equally well) Sprayed the inside of the chamber with 3% H2O2 solution and filled the reservoir bottom with 1% H2O2. My humidity meter returned 92 to 94% readings on several occasions. The meter might have been achieving saturation at that point, but I was only trying for 90% or above so - objective achieved. Even heating should also help sustain even humidity. Clean and scrub hands. Snap razorblade in half lengthwise and clean with alcohol using cotton swab or q-tip. Cut Angle First attempts, In fact the whole first round, were made using cuts at approximate 30 deg angle. I switched to straight or 0 degree cuts for Round 2. Much easier and success rate was improved. Watering – Make sure you are well watered 48 hours in advance. You do not want to be in a position of having to water for the first week in the chamber. The general consensus is that water pressure upwards from the roots can cause graft failure. Even having followed procedure, I observed water weeping from the graftline on several where I did not have great glue coverage. Misting the Plants – Many do, I did not. Having a water reservoir in the chamber and spraying the chamber sides provided an excellent humidity level and I did not see any benefit in doing so. Trimming excess scion foliage – I trimmed at grafting time. Suggested to trim a day or so in advance to allow the plant recovery time before grafting. I did not have time to do the advance trim, but I think it might be a good idea. Grafting Jig – This was a great idea. Simple way to hold the rootstock steady and hold the scion in place freeing you up to apply the glue and making sure you have good contact. Anne made hers by using two 3 mm spring clips glued to a tongue depressor. Anne’s Jig  I modified it slightly in that I wanted to be able to adjust the height and the gap between the 2 clips. Both a 5 mm knitting needle and a similar dimensioned craft stick both fit into a spring grafting clip. My Jigs  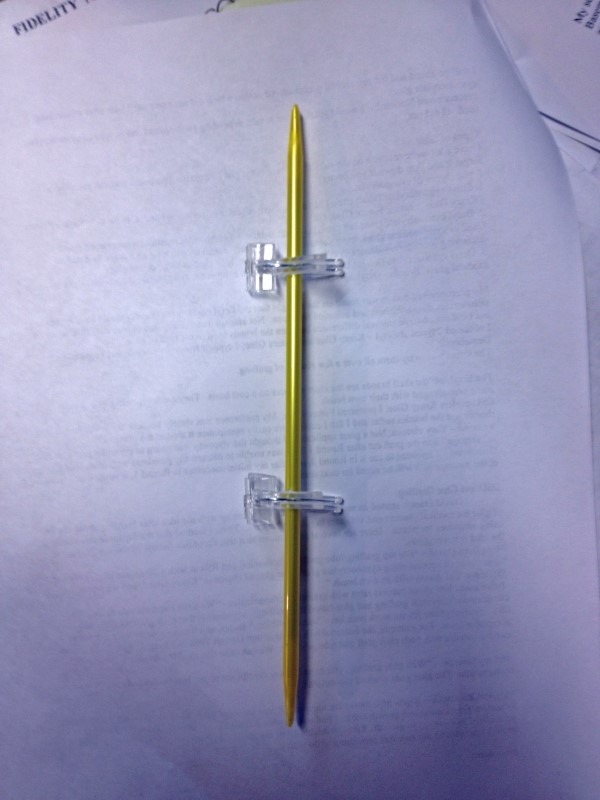 My overall use of the grafting jig was defeated by my use of potting blocks though. Smaller blocks generally don’t like you sticking anything in them without crumbling occurring. I managed 2 grafts using the grafting jig in Round 1 before admitting defeat and being forced to “free hand” the rest. I think it would work great using cups though. The 2 grafts made with the jig were both successful. Don’t be afraid to trim and re-trim your cut lines to get them to match. After gluing several I wasn’t happy with the look of the graft and re-grafted them, by trimming off above and below the glue line. Put it aside and wait for the glue to dry first though. I ruined a few blades by not waiting. I made grafts both above and below the cotyledons. 2 reasons – 1. Trying to match stem sizes as closely as possible, and 2. 2. Just to see what would happen. Did not observe any real differences other than having to trim a few Maxifort suckers that would appear when I grafted above. Grafts made – into the Humidity Chamber on the heat pad. No light for 48 hours. Chamber is covered with a black garbage bag and I would pop the lid for air exchange every 12 hours or so. After 48 hours, bag removed and they went on a 14 hour light cycle under the fluorescent lights with the rest of my plants. Note – I used an open weave carpet pad to supply reduced light to one chamber for several days after the blackout period, and did not observe any differences - will not bother with this step again. Same routine for the next 7 days. Pop the lid every 12 hours, otherwise left alone. Day 8 – Slowly start introducing them to lower humidity. I would take the humidity dome off for an hour here and there for a few days. At this point I thought it was fairly obvious which grafts would make it and which would not. In fact, I think you can tell after 4 or 5 days which are going to make it. 3 or 4 additional days of reduced humidity and they were off the heat mat and into the regular environment. I probably could have increased my success rate by keeping the weaker looking grafts in the chamber for a longer time period, but I had more successes than I needed so I wasn’t interested in trying to “help” any along and instead ruthlessly voted them off the Island. I did re-graft 2 weak looking ones from the end of Round 1 and had 1 take and survive Round 2. Additional Notes, Conclusions and Observations The glue had an odd effect in that is stripped out the color of the stem wherever it made contact in the first week. That change disappeared over time.  I also believe I had “seepage” into the graft on more than a few as it caused a “withering effect” on a few grafts that extended above and in some cases below the graft line. In places an inch or more. Long term effects if any – TBD. (Edit note – no long term effect that I could discern)  Glue versus clips – The one benefit that is clear to me – You do not have to worry about a “window” of grafting opportunity. Nor do you need multiple clip sizes on hand. Round 1 grafts were made with the stem size right around 2 mm and in Round 2 they all easily exceeded the 2mm clip size. It was much easier to glue graft with larger stem diameters and I think this contributed to my Round 2 success rate. Results This was definitely both a fun and interesting project. The overall question ‘Was it worth it?” - Yes. Season Observations I grew side by sides, grafted versus non-grafted of the following: Cherokee Purple, Stump of the World, Vorlon, Japanese Trifele Black, Goose Creek Plant Size– Grew to essentially the same size. Tomato Size – Grafted tomatoes were for the most part, larger by 1 to 3 ounces. I had multiple grafted CP, Vorlon and Stump come in over 16oz.  Also of note were instances of drastic change in shape, in particular with respect to JTB. All the tomatoes on the left were from grafted JTB.  Grafted Cherokee Purple also in several instances produced incredibly uniform color and shape with less cracking. It did not happen in all instances, and I had plenty of cracking on grafted CP tomatoes as well. Grafted CP on the left.  Taste and Flavor – I cannot say that the grafted were a clear winner in this category. The tomatoes that were “good to great to spectacular” came from both sets of plants. Volume – Grafted plants produced more tomatoes. This was clearly noticeable towards the end of the season. Winner – Grafted plants. Not a huge margin of victory, but a clear winner. Link to my 2013 Season Snapshot post |
|
|

|
|
|
#2 |
|
Tomatovillian™
Join Date: Nov 2011
Location: Long Island NY
Posts: 1,992
|
|
|
|

|
|
|
#3 |
|
Tomatovillian™
Join Date: Mar 2011
Location: Raleigh, NC
Posts: 1,448
|
Nice idea on the jig! I don't use the spring clips but the silicone clips could be used similarly. I've free handed my superglue grafts and it's a bit tedious. Thicker stems are much easier to work with.
__________________
Blog: chriskafer.wordpress.com Ignorance more frequently begets knowledge: it is those who know little, and not those who know much, who so positively assert that this or that problem will never be solved by science. --Charles Darwin |
|
|

|
|
|
#4 | |
|
Tomatovillian™
Join Date: Nov 2011
Location: Long Island NY
Posts: 1,992
|
Quote:
Eliminates the need for a real steady hand. Doesn't help with the tedium though! |
|
|
|

|
|
|
#5 |
|
Tomatovillian™
Join Date: Mar 2011
Location: Raleigh, NC
Posts: 1,448
|
Yah, that's kind of why I gave up on superglue...or maybe I need to drink less coffee....
__________________
Blog: chriskafer.wordpress.com Ignorance more frequently begets knowledge: it is those who know little, and not those who know much, who so positively assert that this or that problem will never be solved by science. --Charles Darwin |
|
|

|
|
|
#6 |
|
Tomatovillian™
Join Date: Jul 2009
Location: Ontario, Canada
Posts: 692
|
Fantastic!!
 You are to be congratulated for taking the time and trouble to document all the steps you made. First class. Thanks again for sharing. 
|
|
|

|
|
|
#7 |
|
Tomatovillian™
Join Date: Sep 2010
Location: Sacramento CA
Posts: 288
|
James,
Great recordkeeping and write up. Thank you. "Plant Size– Grew to essentially the same size." Did you prune your plants? My Maxifort RS grafts were very vegetative in 2013 and I wished I had pruned them more. Nice jig. Would positioning the clips closer together make a difference in a tighter alignment prior to gluing? Please don't wait until 2015 to publish your 2014 grafting experience! Thanks, Rick |
|
|

|
|
|
#8 |
|
Tomatovillian™
Join Date: Oct 2011
Location: Durhamville,NY
Posts: 2,706
|
Very Interesting read. You talk about breaking the razor in half lengthwise. I take it you where using a double edged blade. Why not just use a single edged blade or a scalpel for that matter?
|
|
|

|
|
|
#9 |
|
Tomatovillian™
Join Date: Nov 2011
Location: Long Island NY
Posts: 1,992
|
Beeman -Thanks!
Rick- Pruning - not regularly. I prune the bottom of the plants at season start, and for airflow midseason. That's about it, other than to take off diseased or dead growth once in a while. I didn't really get super explosive plant growth. What I assume were limiting factors - Bad weather year, roots being limited in the tubs, different fertilizer and schedule. The jig- It might. Worth trying for sure. I viewed it as more of an extra hand than to apply pressure and I only got to do a couple with it. I am going to attempt to post "real time" this year. Otherwise it just might take me a year! Might be starting some seed for grafting next week. Doug - The Wilkinson Sword Razor Blades were recommended as being super thin. Definitely thinner than the single edge blades and sharper. Split in half they were easy to manipulate. I don't see any issues with using a scalpel or the single edge though. |
|
|

|
|
|
#10 |
|
Tomatovillian™
Join Date: Dec 2012
Location: Utah
Posts: 693
|
Great job! Thanks for sharing. Does the glue restrict growth where it attaches, does it stretch as the plant grows or does it separate in places and the plant fill in?
|
|
|

|
|
|
#11 | |
|
Tomatovillian™
Join Date: Nov 2011
Location: Long Island NY
Posts: 1,992
|
Quote:
Thanks! I would say the glue was more restrictive until it started to crack due to plant growth. No flex really in Krazy or Future Glue. It sort of scabs right off once they gain some size. Quite the mixed bag on how the grafts looked before planting. No obvious side effects, but it was on my radar screen to take a closer look at this season. |
|
|
|

|
|
|
#12 |
|
Tomatovillian™
Join Date: Feb 2010
Location: Merced, CA
Posts: 832
|
Hi James,
Thanks for the great post - I've been looking forward to it! Great info, photos, documentation, and conclusions/summary  . . And thanks for the "credit" on the jig (though not necessary). The clips on the knitting needle are a great idea, if you're going the "jig-route." I tied a couple of the spring clips that fit snuggly (but not too snug) on a number 9 knitting needle so that you can slide them around to reposition, while the clips still work fine and stay put wherever you position them. So, in theory, you could have your scion in one clip and the rootstock in the other, then slide the clips so that the matching stem diameters are aligned for a cut. (Looking down from the top, the clips would be offset a few degrees so you'd be able to have the stems line up next to each other. After making the cut, you'd slide the clips apart, then reposition them to match up the cut for the glue joint (or, I suppose you could just put a silicon or other grafting clip on the graft seam). In a way it would serve as a cutting jig as well as a gluing jig. (Hope this is clear - a little hard to explain without photos). Too bad your growing cubes didn't work out with the jig. I think you had a really great success rate - that will probably be even better this coming season. Very interesting how some of the fruits seem to have been affected by the grafting - particularly the JBT! Also, very interesting what Dr Francis had to say -particularly about the incompatibility issues (given that we briefly discussed this on the no-root grafting thread). Thanks for getting in touch with him and doing all the research you did on the subject!! Looking forward to reports on your 2014 projects and results now  ! ! Anne (who may give glue grafting another try) |
|
|

|
|
|
#13 |
|
Tomatovillian™
Join Date: Nov 2011
Location: Long Island NY
Posts: 1,992
|
Thanks Anne! Took me long enough to get that posted didn't it?
The Jig - Your explanation is good. I think the jig is going to come back into play for me. Definitely going to try some of Delerium's methods using glue and use DE. Hopefully the DE will be strong enough to support the jig in a Solo cup. If not, back to freehanding..... JTB - I was getting the weird shape changes with that one on the grafted plants all season. I have no explanation. Not growing that one again though so no repeat test. Looking forward to your results with the vertical graft as well. Have you started yet? |
|
|

|
 |
|
|
